What is an imported medical device?
An imported medical device refers to any device manufactured outside mainland China and intended for marketing within the country.
It must be registered or filed in accordance with the Regulations on the Supervision and Administration of Medical Devices (State Council Decree No. 739), and relevant NMPA regulatory rules.
—
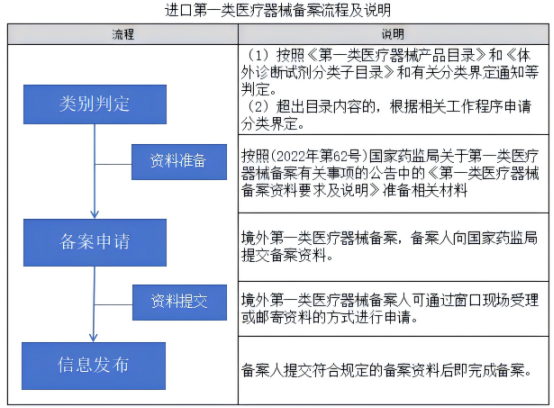
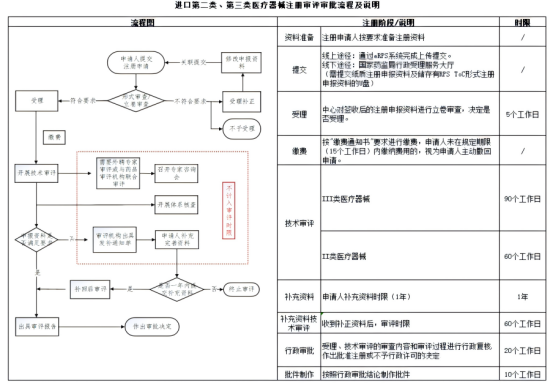
Note: Imported Class I devices follow a filing process, while Class II and III devices require formal registration and technical review by CMDE.
Filing vs. Registration
Filing: For low-risk (Class I) devices – a simplified notification procedure
Registration: For medium- and high-risk (Class II & III) devices – full evaluation required
Who can apply?
Overseas manufacturers must appoint a China-based legal agent to handle registration, submission, and communication with authorities.
How to Submit Registration or Filing Documents for Imported Medical Devices?
To market a medical device in China, the product must be:
Defined as a medical device under Chinese regulations, in line with the Regulations on the Supervision and Administration of Medical Devices (Decree No. 739), and Manufactured outside of mainland China, intended for import.
Required Documentation
If the product is already classified as a medical device in the country of origin:
The applicant must provide:
A marketing authorization certificate or equivalent proof of legal sale in the originating country
A manufacturer’s qualification certificate, proving compliance with GMP or QMS (e.g., ISO 13485)
If the product is not considered a medical device overseas, but is classified as one in China:
The applicant must provide:
A declaration letter stating the product is not regulated as a medical device abroad
Relevant technical documentation and proof of legal marketing (as applicable)
Device Class | Submission Type | Technical Review Body | Notes |
Class I | Filing with NMPA | NMPA completeness check only | Simple administrative review |
Class II | Full registration | CMDE technical + NMPA admin | PTR, testing, CER usually required |
Class III | Full registration | CMDE technical + NMPA admin | Higher scrutiny, clinical data essential |
Class I: File with NMPA (no technical review)
Class II & III: Submit to NMPA → CMDE evaluation → receive registration certificate
Registration work flow:https://www.cmde.org.cn/sqrzc/zxfw/lcjteng/index.html
Evaluation work flow: https://www.cmde.org.cn/sqrzc/zxfw/jsspeng/index.html
We'll help you navigate each step — from classification and testing to technical file submission.